Make Your Dream Come True in Reality
Medicoz Community is the specially evolved cohesive force of leading educationalists, having the vast experience in teaching thousands of aspirants of MEDICAL PG ENTRANCE EXAMINATIONS. Our Experience, Expertise and in depth understanding of latest examinations patterns thoroughly blends demands of todays students. WE Adopt the Modern methodology of teaching for those DEDICATED aspirants who are preparing for Medical exams.
Our ideology at Medicoz Community is to provide the students with all Possible inputs to make them realize their True Potential and further enhance it to Touch the Highest level of excellence. It is to be noted that our Organization is with FULL TIME DEDICATION of our educators in different streams. Our one of the most important specialty is to upgrade the techniques and concepts and to teach even the most difficult Concepts Crystal Clear.
Medicoz Community is Highly dedicated to Provide Quality Medical Education in Best and Easiest Possible ways.

Our Features
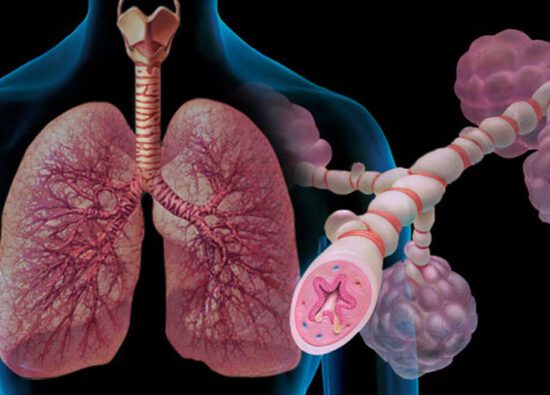
Fully Updated QBank and Test series
With all New Clinical Scenario based questions and we follow 60-30-10 Approach where importance is Given for 60% clinical, 30% Application based and 10% one liners which follows the latest exam pattern. Test series designed and curated to test you with the questions that are on Par with real exam Questions and Generates ALL INDIA RANKS. Helps to get desired score and Top Ranks in Final exams.
LIVE Scheduled Classes by top faculty
Medicoz Community brings you the Live Scheduled classes by Top Subject Experts which helps you to get more engaged with Concepts with Help of interest binding study materials which is designed to boost the Power of remembrance Provided the soft copy of notes during the entire lecture by our Experts. Our faculties are here to take you towards concept based learning.
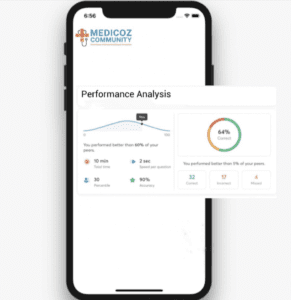
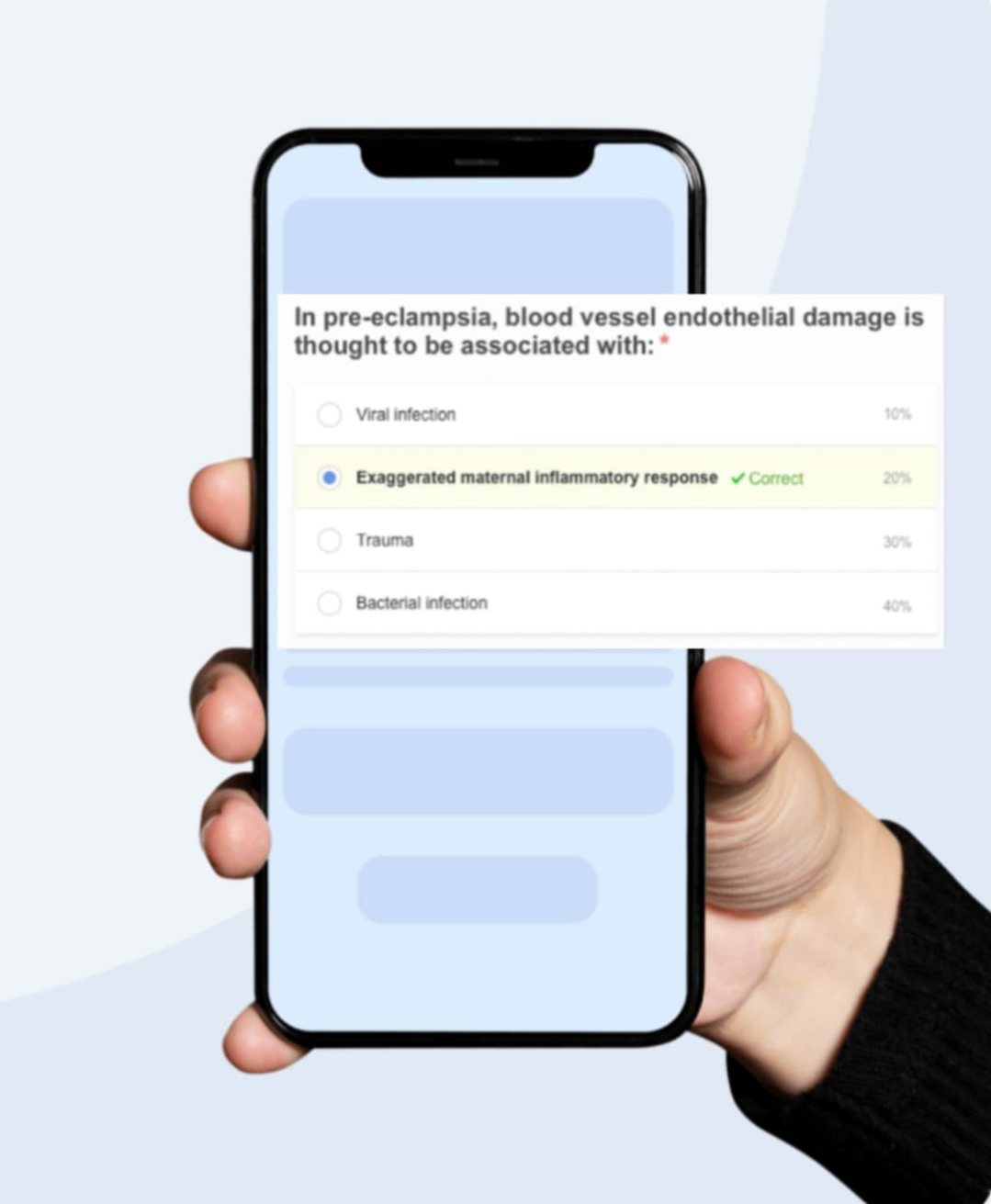
Detailed analysis and solutions
What Makes Medicoz Community Extraordinary?
Importance Given to Concept based learning
Retains more than 40% of what you Read
Makes you Exam Ready
Our Contents are Error free and Evidence Based
Largest Pan-India Test series
Learn using the All New QBank and Test series
Our Location
Mangaluru - 583230Email Address
info@medicozcommunity.comCall Us Free
+91 97407 59591Are you Ready for a Better, more Productive in Your Study?
Lifestyle Modification in management of Hypertension
NEET PG 2023 "General Surgery" Recall Session
Dr. Sowjanya | Medicoz Community
Interesting divs
Viral Meningitis in Paediatric Patient
Case Presented by : Situpriya Rout , 2nd Year MBBS , AIIMS Kalyani Patient Information Name: Master A.R. Age:
Asthma in Obese Patients : Challenges and Outcomes
Presented by : Aarzoo Ali Kaif , 2nd Year MBBS , AIIMS Kalyani ( Medicoz Community Editors Pick )♦♦♦ Patient
Medicoz Community Congratulates NEET PG 2024 Toppers! A Triumph of
The Medicoz community extends its warmest congratulations to all the toppers of the NEET PG 2024 examination. Your exceptional performance